What Is the Temperature Increase of 4.0 Kg of Water
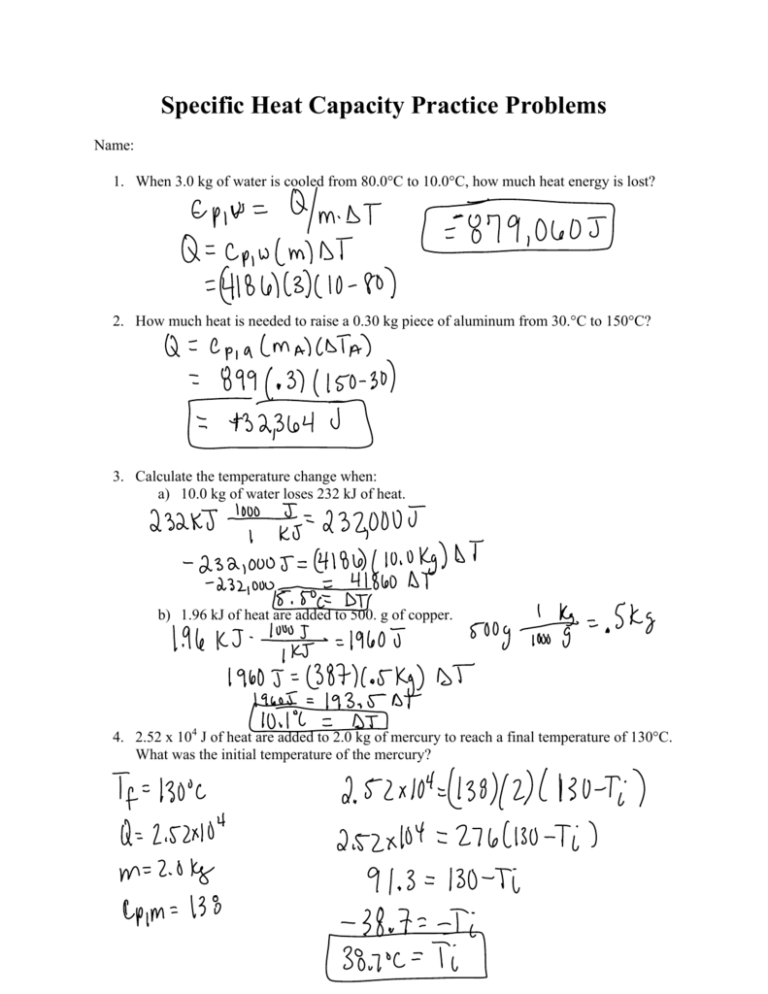
66C In order to be able to solve this problem you need to know the value of waters specific heat which is usually given to be 418Jg C. C w 4 186 JkgC a.

Specific Heat Of Water Video Khan Academy
Time 10 min 10 60 600 s.

. Cp 4186 Jkg C Help. Calvin times to change in temperature of the water which you wish to solve for Delta T So we can simply rearrange this equation. Cw 4 186 JkgC a.
This is the amount of heat required to raise 1 gram of that substance by 1C. Experts are tested by Chegg as specialists in their subject area. What is the increase in the initial energy per kilogram of water at the bottom of a 145 m waterfall assuming that all of the initial potential energy is transferred as heat to the water.
View solution A water fall is 1 2 6 meters high. What is the temperature increase of 40 kg of water when heated by an 800-W immersion heater for 10 min. 14 C ____ 30.
Now using the equation of specific heat Q mcΔT. The use of fiberglass insulation in the outer walls of a building is intended to minimize heat transfer through. Mass of water 40 kg.
This will require 2669 kJ of heat energy. See the answer What is the temperature increase of 40 kg ofwater when heated by an 800-W immersion heater for 10 min. A solar heating system has a 250 conversion efficiency.
Now the equation that establishes a relationship between heat lost or gained and. Up to 24 cash back What is the temperature increase of 40 kg of water when it is heated by an 80 10 2 W immersion heater for exactly 100 min. What is the temperature increase of 40 kg of water when heated by an 800-W immersion heater for 10 min.
Water is heated by 8 10² W 800 W. Who are the experts. The mass of the material m The temperature change that occurs DeltaT The specific heat capacity of the material c which you can look up.
In our example it will be equal to c -63000 J 5 kg -3 K 4200 J kgK. Substituting Pt mcΔT. Question 1 What is the temperature increase of 40 kg of water when heated by an 800-W immersion heater for 50 min.
4 kg 1000 grams1 kg 4000 grams. Since we have one unknown and solve for Delta T and since all these s I units we find that delta t the change in temperature of the water is 231 degrees Celsius. 142245 J What is the temperature increase of 40 kg of water when it is heated by an 80 x 102 W immersion heater for exactly 100 min.
Cw 4 186 JkgC. - Answers Conversion needed. What is the temperature increase of 40 kg of water when heated by an 800-W immersion heater for 10 min.
The temperature of water at the bottom of a large waterfall is higher than that of the water at the top because. This is the typical heat capacity of water. What is the temperature increase of 40 kg of water when it is heated by Q 480 105 J.
What is the temperature increase of 40 kg ofwater when heated by an 800-W immersion heater for 10 min. Should astronomers continue to research about ancient Egyptian astronomy. To calculate the energy required to raise the temperature of any given substance heres what you require.
A Calculate the rate of temperature increase in degrees Celsius per second ºCs if the mass of the reactor core is 160 10 5 kg and it has an average specific heat of 03349 kJkg ºC. If you have problems with the units feel free to use our temperature conversion or. At room temperature is four 018 and thats killer Jewel the K G.
How much thermal energy is needed to raise the temperature of 40 kg of water from 25 degrees Celsius to 75 degrees celsius. Cw 4 186 Jkg x degreesCelsius. A 200-kg copper rod is 5000 cm long at 23C.
C p 4186 Jkg C a. Given mass of water 4 Kg Water is heated to 800 W time of immersion 10 min 10 x 60 600 s using equation of specific heat Q m S ΔT S is the specific heat capacity of water which is equal to 4182 JkgC. The solar radiation incident on the panels is 1 000 Wm2.
What is the temperature increase of 40 kg of water when heated by an 800-W immersion heater for 10 min. Why or why not. A substances specific heat tells you how much heat is required to increase the temperature of 10 g of that substance by 1C.
Calculate specific heat as c Q mΔT. We review their content and use your feedback to keep the quality high. What is the temperature increase of 40 kg of water when heated by an 800 W immersion heater for 10 min.
The solar radiating incident on the panels is 1000 Wm2. And another formula of heat Q Pt now P t m S ΔT 800 x 600 4 x 4182 x ΔT ΔT 29 C. Where c is the specific heat capacity of water 4186 JkgC.
What is the increase in temperature of 300 kg of water in a 100-h. What is the temperature increase of 40 kg of water when heated by an 800-W immersion heater for 10 min. C w 4 186JkgC Best Answer 100 1 rating Given that the mass of the water is m 40 kg The power is P 800.
What is the temperature increase of 40 kg of water when heated by an 800-W immersion heater for 10 min. Cw 4 186 JkgC a. Cw4186 Jkg x degrees C 29 degrees C A solar heating system has a 25 conversion efficiency.
B How long would it take to obtain a temperature increase of 2000ºC which could cause some metals holding the radioactive materials to melt. If half of the potential energy of the falling water gets converted to heat the rise in temperature of water will be. What is the temperature increase of 40kg of water when heated by an 800W immersion heater for 10min.
Cw 4 186 JkgC 29 degrees C Dmitri places one end of a copper rod in a heat reservoir and the other end in a heat sink. Answer is 29C how do you get it.

Solved Question 9 Not Yet What Is The Temperature Increase Chegg Com
No comments for "What Is the Temperature Increase of 4.0 Kg of Water"
Post a Comment